Effect of oral ponesimod on clinical disease activity and MRI-based outcomes in patients with relapsing multiple sclerosis: Phase 3 OPTIMUM study
Abstract
Background:
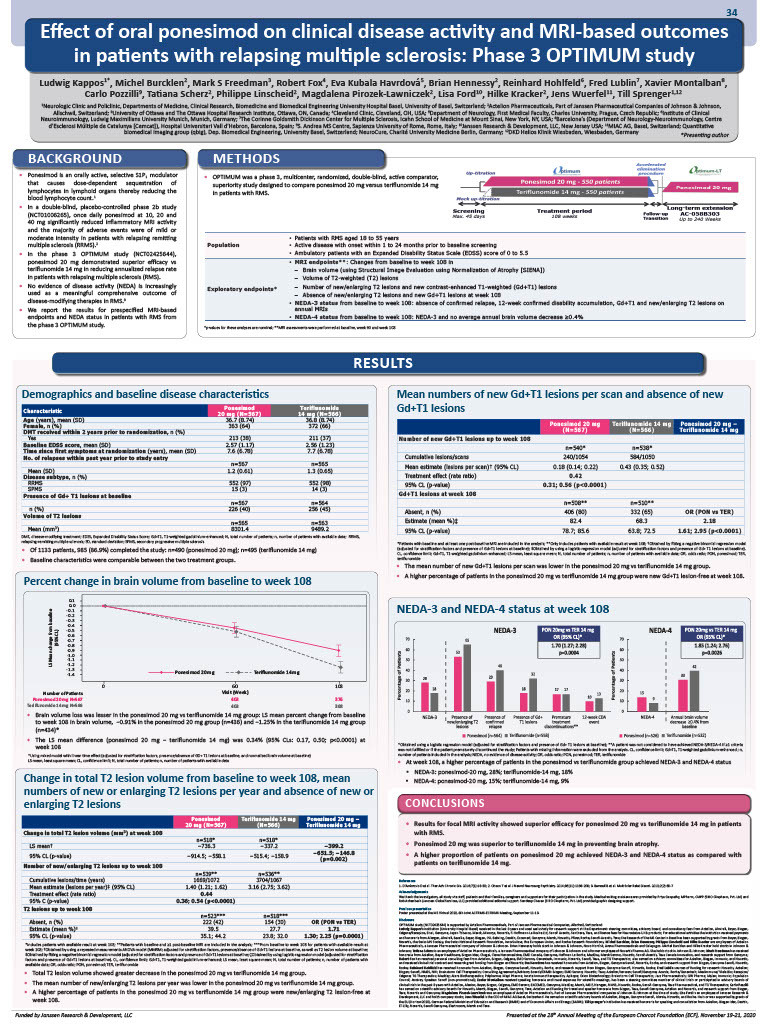
In the phase-3 OPTIMUM study (NCT02425644), ponesimod (PON), a selective modulator of sphingosine-1-phosphate receptor 1, showed superior efficacy vs teriflunomide (TER) in patients with relapsing multiple sclerosis (RMS). Prespecified MRI-based endpoints and no evidence of disease activity (NEDA) status were evaluated.
Methods:
Patients (18-55 years) with RMS (expanded disability status scale scores: 0-5.5) were randomized (1:1) to PON 20-mg or TER 14-mg for 108 weeks. MRI endpoints: percentage change from baseline to week 108 in brain volume (BV), mean number of new gadolinium-enhancing (Gd+) T1-lesions and volume/count of new/enlarging T2-weighted (T2)-lesions. NEDA-3 (absence of confirmed relapse, 12-week confirmed disability accumulation, Gd+T1 and new/enlarging T2-lesions on annual MRIs) and NEDA-4 status (NEDA-3 and no average annual BV decrease ≥0.4%) were evaluated from baseline to week-108.
Results:
985/1133 (86.9%) randomized patients completed the study. MRI findings for PON vs TER, respectively, were: LS mean percent change in BV: −0.91% vs −1.25% (difference:0.34%, 95%-CLs:0.17;0.50, p<0.0001); LS mean difference (PON−TER) for change in total T2-lesion load: −399.2 mm3 (95% CLs:−651.5;−146.8, p=0.002); mean number of new/enlarging T2-lesions per year: 1.40 vs 3.16 (rate ratio [RR]:0.44, 95%-CLs:0.36;0.54, p<0.0001); PON vs TER odds ratio (OR [95%-CL]) for absence of new/enlarging T2-lesions: 1.71 (1.30;2.25, p=0.0001); mean number of new Gd+T1-lesions per scan: 0.18 vs 0.43 (RR:0.42, 95%-CLs:0.31;0.56, p<0.0001); PON vs TER (OR [95%-CL]) for absence of new Gd+T1-lesions: 2.18 (1.61;2.95, p<0.0001). At week 108, 28.2% (159/564) PON vs 18.3% (102/558) TER patients (OR:1.70, 95%-CL:1.27;2.28, p=0.0004) achieved NEDA-3; 15.0% (79/526) PON vs 8.5% (45/532) TER patients (OR:1.85, 95%-CL:1.24;2.76, p=0.0026) achieved NEDA-4. The most common reason for not achieving NEDA-3/ NEDA-4 status was presence of new/enlarging T2-lesions.
Conclusions:
PON showed benefit vs TER for all MRI outcomes including BV loss and a significantly higher proportion of patients achieved NEDA-3 and NEDA-4 status, supporting the effects observed on clinical endpoints.
Effect of oral ponesimod on clinical disease activity and MRI-based outcomes in patients with relapsing multiple sclerosis: Phase 3 OPTIMUM study
Ludwig Kappos1*, Michel Burcklen2, Mark S Freedman3, Robert Fox4, Eva Kubala Havrdová5, Brian Hennessy2, Reinhard Hohlfeld6, Fred Lublin7, Xavier Montalban8, Carlo Pozzilli9, Tatiana Scherz2, Philippe Linscheid2, Magdalena Pirozek-Lawniczek2, Lisa Ford10, Hilke Kracker2, Jens Wuerfel11, Till Sprenger1,12
*Presenting Author
1Neurologic Clinic and Policlinic, Departments of Medicine, Clinical Research, Biomedicine and Biomedical Engineering University Hospital Basel, University of Basel, Switzerland
2Actelion Pharmaceuticals, Part of Janssen Pharmaceutical Companies of Johnson & Johnson, Allschwil, Switzerland
3University of Ottawa and The Ottawa Hospital Research Institute, Ottawa, ON, Canada
4 Cleveland Clinic, Cleveland, OH, USA
5Department of Neurology, First Medical Faculty, Charles University, Prague, Czech Republic
6 Institute of Clinical Neuroimmunology, Ludwig Maximilians University Munich, Munich, Germany
7 The Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY, USA
8 Barcelona's (Department of Neurology-Neuroimmunology, Centre d’Esclerosi Múltiple de Catalunya [Cemcat]), Hospital Universitari Vall d’Hebron, Barcelona, Spain
9 S. Andrea MS Centre, Sapienza University of Rome, Rome, Italy
10 Janssen Research & Development, LLC, New Jersey USA
11 MIAC AG, Basel, Switzerland; Quantitative biomedical imaging group (qbig), Dep. Biomedical Engineering, University Basel, Switzerland; NeuroCure, Charité University Medicine Berlin, Germany
12DKD Helios Klinik Wiesbaden, Wiesbaden, Germany
The poster was previously presented at the 8th joint ACTRIMS - ECTRIMS Meeting, (MSVirtual 2020), September 11-13, 2020.