Cladribine: 14 years brain atrophy and clinical follow-up
Abstract
Background:
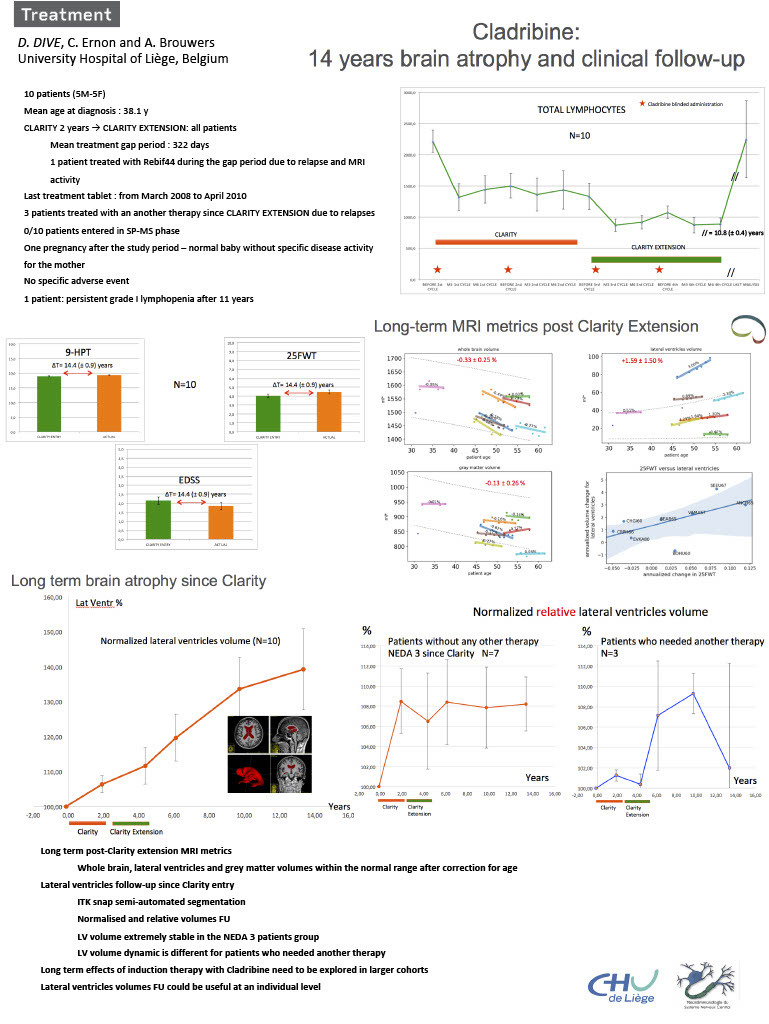
Ten patients were included in Clarity and Clarity extension studies in our center. All of themare still followed and long-term brain atrophy and clinical measurements were evaluated.
Methods:
Multimodal clinical evaluation (EDSS, 9-HPT, 25-FWT) was used throughout the wholefollow-up period. Brain atrophy and T2 lesion load were quantified during the last five yearswith the IcoBrain technology. Lateral ventricles volumes were quantified with a semiautomatedtechnique (ITK-SNAP software) from study entry to the last exam with a meanfollow-up time of 14 years. Total lymphocytes count was evaluated throughout.
Results:
Over 14 years, 7 patients didn’t require additional therapy after cladribine, as they remainedNEDA3 throughout the entire follow-up. Three patients relapsed within 5 years and movedto other treatments. Total lymphocytes were normal at the end of the follow-up period. Nopatient entered in a secondary progressive phase. EDSS, 9-HPT and 25-FWT did not changesignificantly after 14.4 years mean time of follow-up. Annual brain atrophy evolution waslimited and was comparable to that occurring in normal healthy individuals (whole brainvolume -0.33 ± 0.25 % - lateral ventricles volume +1,59 ± 1.50 % - gray matter volume -0,13 ±0,26 %). Evolution of brain atrophy in NEDA3 patients differed from what was observed inpatients who had to be switched to another therapy.
Conclusion:
In our cohort of Clarity patients, initial cladribine treatment was associated with stabilizationof MS in a majority of patients for over 10 years. Clinical stability was associated with longtermbrain atrophy progression in line with what is expected in a healthy population, anddiffered from what was seen in non-stable patients.